News & Trends - MedTech & Diagnostics
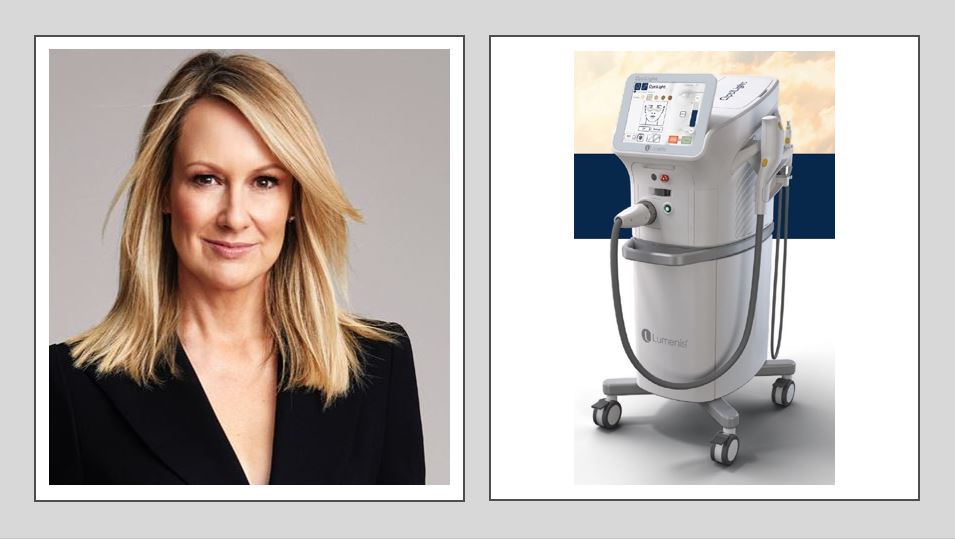
Lumenis to launch first IPL for management of dry eye disease
MedTech News: According to Google Trends, searches for ‘dry eye’ in Australia have increased by 340% since 2005. While age is a factor of Dry Eye Disease (DED), newer risks like increased screen time, longer exposure to air-conditioning, and mask wearing are indicating that DED is becoming prevalent in younger age groups.
Lumenis, the world’s largest energy-based medical device company for ophthalmic, aesthetic and surgical applications and the inventor of intense pulsed light (IPL) technology, has announced its newest IPL device for improving signs of dry eye disease due to meibomian gland dysfunction (MGD).
In January 2020, Optometry Australia reported around 77% of Australians have suffered from dry eye symptoms, yet only 26% have visited an optometrist to discuss treatment options. Current literature even suggests that up to 86% of dry eye patients demonstrate signs of MGD.
Lumenis’ clinical trial showed that Lumenis IPL with patented Optimal Pulse Technology (OPT) significantly improved tear breakup time, meibum quality, and meibomian gland expressibility.
Subsequent to the FDA approval and TGA listing, Lumenis is launching OptiLight, a bright solution for dry eyes. OptiLight with Lumenis’ patented OPT technology is designed for a consistent, precise, and controlled light-based treatment of signs of dry eye disease.
“In my clinic we deal with dry eye disease every day and have found that in order to make a meaningful impact on the disease, we need to address the underlying inflammation. Lumenis’ OPT technology helps us to address inflammation, as shown in published clinical trials, which improves the signs and symptoms of dry eye disease due to MGD. Lumenis’ OPT is an essential tool in our dry eye toolkit,” said Dr Jason Holland, Lead Clinical Optometrist at The Eye Health Centre in QLD.
Lumenis Country Manager, Richard New, commented “Lumenis has launched many ‘firsts’ in eye care, and we never stop innovating – which is why we are proud our new OptiLight device with patented OPT technology is the first and only such device to receive FDA approval for improving signs of dry eye disease. As a business we are committed to elevating dry eye management to further improve the quality of life for millions of Australian patients.”
Australian journalist Melissa Doyle said “Having been recently diagnosed with Dry Eye Disease after more than 10 years of enduring irritating symptoms, Melissa represents the many thousands of Australians unaware that the sore, red, watery, and puffy eyes they are living with is not the result of allergies, eye strain or some other temporary condition – but a serious chronic and progressive eye condition affecting hundreds of millions of people around the world.
“For over a decade, I have endured gritty, watery and puffy eyes that have impacted my day-to-day life significantly,” reveals Melissa. “It was common knowledge on the set of Sunrise that my eyes would flair up and cause me problems, and there were even days when my eyes were so bad, I couldn’t go to work”.
“I was always told the problem was likely allergies, so have endured just about every allergy test under the sun, however after a recent visit and testing with Dr Jim Kokkinakis at The Eye Practice in Sydney, I have now been diagnosed with Dry eye disease due to ocular rosacea.”
Dr Kokkinakis added “Ocular rosacea is a very common cause of dry eye disease, highly under diagnosed and therefore often inappropriately treated, as in Melissa’s case”.
Lumenis will unveil the new OptiLight technology for the Australian and New Zealand market during a planned virtual launch in November 2021.
News & Trends - MedTech & Diagnostics
Bariatric surgery trumps Novo Nordisk’s Wegovy in cost-effectiveness and durability
MedTech & Diagnostics News: Bariatric surgery emerges as cost-effective, boasting superior and enduring weight loss outcomes over a five-year span […]
MoreNews & Trends - Pharmaceuticals

Aussie digital health company hits new milestone in AstraZeneca partnership
Pharma News: Fewer than 50% of asthma patients adhere to their prescribed preventative medications. An Australian digital health company has […]
MoreDigital & Innovation

Medical drone to reduce health equity gaps in rural and remote Australia
A specialised medical drone which increases accessibility to essential health services such as pathology, medicines, and telehealth services in rural […]
More