News & Trends - Biotechnology
Will the eyes of global pharma be fixed on this local biotech?

Biotech News: A Perth-based biotech company is spearheading the clinical trial for a pioneering medicine aimed at minimising brain tissue damage following a stroke.
Ten (10) hospitals across Australia, including Royal Melbourne, Princess Alexandra, John Hunter, Sir Charles Gairdner Hospital, and Fiona Stanley Hospital, will be running Argenica Therapeutics’ phase II trial for ARG-007.
Western Australia Medical Research Minister, Stephen Dawson, expressed his enthusiasm for the potential impact of this novel drug, highlighting its significance for stroke patients.
“Global pharmaceutical companies will be watching this trial very closely as this drug could be effective against other illnesses, such as Alzheimer’s and Parkinson’s disease. The Cook Government is proud to be able to financially support innovative ideas and medical research masterminded here in Perth,” Minister Dawson emphasised.
The SEANCON trial focuses on assessing the safety of ARG-007 in acute ischemic stroke (AIS) patients. Success in proving the drug’s safety in AIS patients will pave the way for Argenica to advance to a pivotal Phase 3 trial and potentially attract the attention of global pharmaceutical companies, such as Boehringer Ingelheim and Biogen.
Key stakeholders in stroke care united in October last year to establish ambitious benchmarks to ensure that all Australians have access to world-class stroke treatment. The Stroke Foundation, Stroke Society of Australasia, Australian Stroke Clinical Registry, and the Boehringer Ingelheim’s Angels Initiative combined their wealth of expertise at the National Stroke Targets Roundtable. Their collective efforts culminated in the development of a visionary set of objectives that Australian medical facilities aim to achieve by 2030.
Argenica’s ARG-007 holds promise in improving patient outcomes by safeguarding brain tissue from irreversible damage until normal blood flow can be restored. This potential ‘wonder drug’ is projected to not only reduce further brain injury but also mitigate long-term disability for stroke survivors.
The trial’s patient selection, exclusively enrolling those with diagnosed large vessel occlusion (LVO) strokes eligible for endovascular thrombectomy, ensures a focused approach for evaluating trial endpoints. LVO strokes, constituting nearly 40% of all acute ischemic strokes, are responsible for 60% of post-stroke dependency and a staggering 90% of mortalities after stroke, making them the most devastating type of stroke.
A/Prof Laetitia de Villiers, Interventional Neuroradiologist, Gold Coast University Hospital and Maurice Ben-Mayor, President of Stryker South Pacific discussed that despite clinical guidelines less than 7% of ischaemic stroke patients in Australia receive endovascular thrombectomy in the public healthcare system.
While anticipation surrounds the SEANCON phase II trial, researchers caution that successful progress may still take 5 to 8 years before ARG-007 becomes available for patients. Despite the timeline, the potential impact on stroke care and post-stroke outcomes is significant, given the substantial economic costs associated with treating stroke victims, estimated to reach $183 billion by 2030.
Argenica’s ambitions extend beyond stroke treatment, with researchers exploring the drug’s potential to halt or slow the progression of Alzheimer’s disease. The company received a $419,000 grant from the Cook Government’s Future Health Research and Innovation Seed Fund Program to explore non-invasive administration routes for ARG-007, expanding its application into Alzheimer’s treatment.
 In reimagining healthcare across the entire patient journey, Health Industry HubTM is the only one-stop-hub bringing the diversity of Pharma, MedTech, Diagnostics & Biotech sectors together to inspire meaningful change.
In reimagining healthcare across the entire patient journey, Health Industry HubTM is the only one-stop-hub bringing the diversity of Pharma, MedTech, Diagnostics & Biotech sectors together to inspire meaningful change.
The content on Health Industry Hub is copyright protected and should only be accessed under individual user licenses. To subscribe, please click here and visit T&Cs here.
Human Resources

Medtronic HR Director deep dives into shifting workplaces and leadership trends: International HR Day
People & Culture: Coinciding with International HR Day, Karen Newell, Senior HR Director for ANZ at Medtronic, joined Health Industry […]
MoreNews & Trends - MedTech & Diagnostics
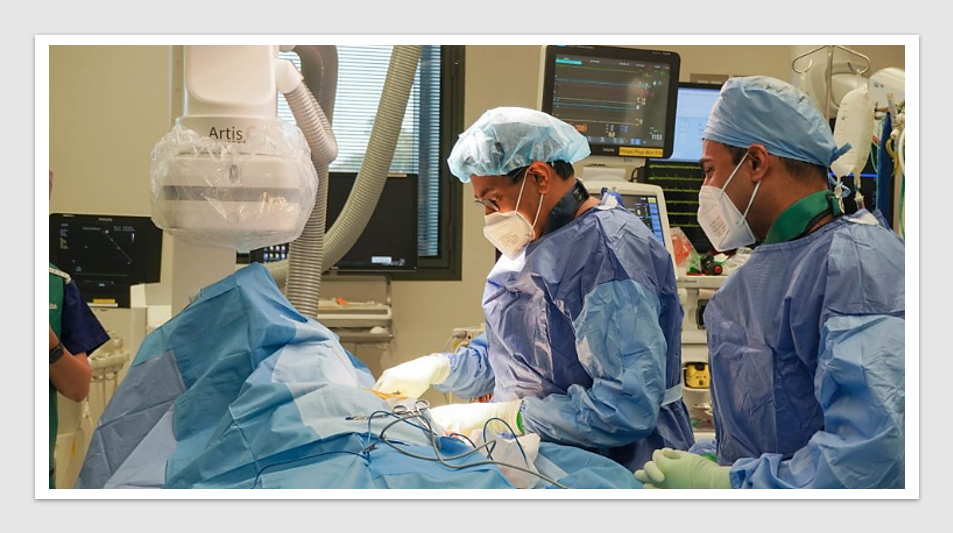
Aussie medtech reveals new data on next-gen pulsed field ablation system for atrial fibrillation
MedTech & Diagnostics News: An Australian-based medtech company announced promising results from the first-in-human study for its pulsed field ablation […]
MoreNews & Trends - MedTech & Diagnostics

Federal budget delivers ‘mixed news’ for radiologists
MedTech & Diagnostics News: The Royal College of Australian and New Zealand Radiologists (RANZCR) and the Interventional Radiology Society of […]
MoreNews & Trends - Pharmaceuticals

AstraZeneca, BMS and Noxopharm address findings of inquiry report into rare cancers
Pharma News: The Senate Community Affairs References Committee has released its inquiry report into Equitable access to diagnosis and treatment […]
More