News & Trends - Biotechnology
BCG anti-TB vaccine trial for COVID-19 protection
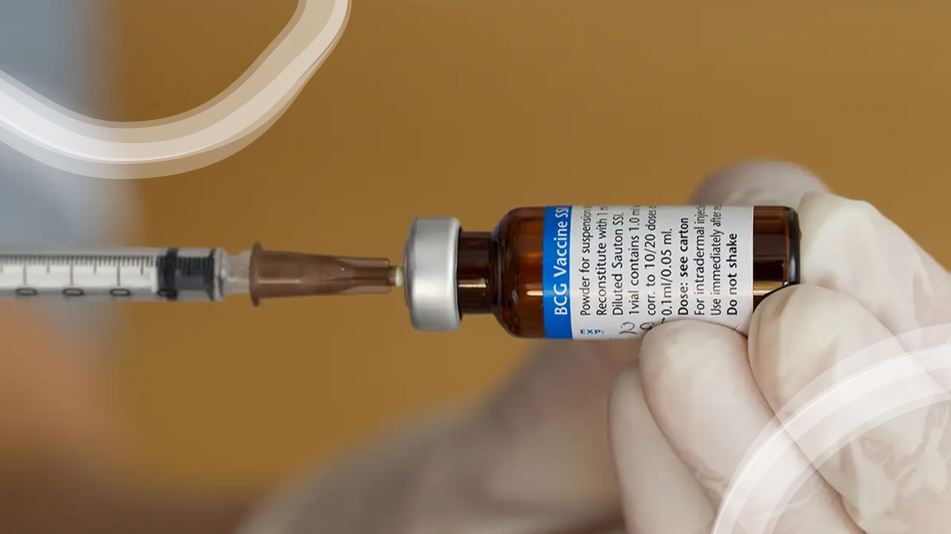
Five hundred front line healthcare workers and paramedics in NSW will join an international trial, to discover whether the tuberculosis (TB) vaccine reduces the impact of COVID-19.
A number of Australian healthcare workers have been infected with coronavirus already, while ongoing personal protective equipment (PPE) shortages have left doctors and nurses vulnerable.
Health Minister Brad Hazzard said he is delighted the NSW Health Services Union (HSU) is supporting the trial with a $350,000 contribution and that so many health staff are taking part.
“This virus is not going away any time soon, so until we have a proven vaccine, trials like this alongside daily testing and COVID-safe behaviours, are vitally important,” Mr Hazzard said.
“Our front line health workers risk exposure to COVID-19 every day, so the sooner we can find a breakthrough and fingers crossed it is the BCG anti-TB vaccine, the better for all of us.”
HSU NSW Secretary Gerard Hayes said the decision to help fund the study was a straightforward one because if the vaccine proves successful, the benefits will be enormous.
“We have to use every tool available to protect our cleaners, security officers, paramedics, therapists and other hospital workers against the severity of COVID which is why we are so enthusiastically supporting this trial,” Mr Hayes said.
“It may seem unusual for us to fund a program such as this, but we know our duty of care to members extends beyond wages and conditions to include their health and wellbeing.”
Dr Rama Kandasamy, Staff Specialist in Immunisation at Sydney Children’s Hospitals Network said the 12 month trial will be rolled out in several hospitals over the coming weeks.
“Workers have already been vaccinated at The Children’s Hospital Westmead and staff at Westmead Hospital, Sydney Children’s Hospital, Randwick, Prince of Wales Hospital and St Vincent’s Hospital will follow,” Dr Kandasamy said.
“Half the participants will receive the BCG vaccine and the other half will receive a placebo and they will be monitored to see if they contract COVID and the severity of their symptoms.
“This trial sets the scene for us to be at the forefront of COVID-19 specific vaccine trials and the results could be the key to providing at-risk groups early protection.”
The BRACE trial was initially launched by Murdoch Children’s Research Institute (MCRI). NSW healthcare workers and paramedics are among 10,000 across Australia along with other countries including Spain and The Netherlands, to participate in the trial.
News & Trends - Biotechnology

AusBiotech appoints new CEO: Former Sanofi corporate affairs and sustainability leader takes the helm
Biotech News: AusBiotech, the nation’s leading industry body for the biotech sector, has named former leader at Sanofi, Rebekah Cassidy, […]
MoreNews & Trends - MedTech & Diagnostics

Federal government invests in Siemens Healthineers scanner to ‘reduce wait times’ for cancer diagnosis
MedTech & Diagnostics News: The Albanese Government is investing $12 million through the 2024–25 Budget, to purchase and install a […]
MoreNews & Trends - MedTech & Diagnostics
Cardiac device benefits face more cuts, while technical services remain secure in the short term
MedTech & Diagnostics News: Starting from July 2024, Cardiac Implantable Electronic Devices (CIED) listed on the Prescribed List (PL) will […]
MoreNews & Trends - Biotechnology

CSL’s world-first gene therapy heads for MSAC evaluation
Biotech News: CSL’s world-first gene therapy for haemophilia B is scheduled for consideration at the upcoming Medical Services Advisory Committee (MSAC) […]
More