News & Trends - MedTech & Diagnostics
World-first Aussie medtech innovation for gastrointestinal disorders

MedTech & Diagnostics News: A strategic investment has been made in a pioneering Victorian medical technology company that has unveiled an innovative medical device to diagnose functional gastrointestinal disorders, including irritable bowel syndrome (IBS) and functional dyspepsia. These conditions, known for their complexity, impact almost 40% of the Australian population.
Atmo Biosciences’ gas-sensing capsule is comparable in size to a standard vitamin pill. Unlike current invasive approaches such as endoscopy, or other diagnostics tools such as breath tests which can be inaccurate or reliant on trial and error, this innovative capsule offers direct insights into microbiome function of the gastrointestinal tract. When ingested, this capsule has the capability to precisely measure gases produced by the microbiota, all while allowing patients to maintain their daily routines. This data is then relayed to Atmo’s cloud platform for analysis.
Early research indicates that this capsule is nearly twice as accurate as conventional breath tests when diagnosing small intestinal bacterial overgrowth (SIBO). This heightened accuracy is anticipated to facilitate targeted treatments, provide early symptom relief, and ultimately lead to a reduction in healthcare costs.
Grant Dooley, CEO of Breakthrough Victoria, expressed enthusiasm about investing in Atmo during this pivotal stage of growth. He said “Atmo’s gas-sensing capsule is a world-first innovation developed and manufactured in Victoria with the potential to help millions of people suffering gut disorders around the world. We’re excited to invest in Atmo during such an important stage of growth, including a pivotal clinical study, ramp up of manufacturing and new investment round. The company has enormous potential for growth at a global scale and we look forward to working with them to help achieve these goals.”
The company’s initial clinical application involves utilising the capsule as a diagnostic tool for motility disorders, with a pivotal clinical study currently underway in both Australia and the United States. Future applications are expected to encompass the capsule’s use as a diagnostic tool for SIBO, a condition associated with symptoms such as abdominal pain, gas, and bloating.
The capsule is also undergoing clinical trials through partnerships with independent research organisations, exploring its potential in diagnosing irritable bowel syndrome (IBS), inflammatory bowel disease (IBD), and liver disease.
This cutting-edge technology traces its roots back to its inception at RMIT University in 2011, subsequently evolving into the startup company Atmo and receiving critical support for commercialisation from healthtech innovation enterprise Planet Innovation.
Atmo Biosciences CEO, Mal Hebblewhite, conveyed his delight over Breakthrough Victoria’s strategic investment in the company.
“This is a very exciting time for Atmo as we progress towards market entry with our first product to evaluate motility disorders and look to scale up our manufacturing capability to meet expected demand for the product. Gastrointestinal disorders are a big problem in Australia and around the world, with patients suffering debilitating symptoms such as nausea, vomiting, diarrhoea and abdominal pain. Unfortunately, due to shortcomings with current diagnostic methods, and despite frequent visits to the doctor, it’s estimated about 30% of sufferers remain undiagnosed or are misdiagnosed. Atmo is hoping to change that,” he commented.
The investment from Breakthrough Victoria comes as a share purchase from Planet Innovation, adding to Atmo Biosciences’ previous grants from MTPConnect’s BioMedTech Horizons program, ANDHealth and the company’s successful funding rounds.
Looking ahead, Atmo Biosciences is gearing up for its Series C investment round in 2024, set to fund critical commercialisation efforts and manufacturing capabilities.
 In reimagining healthcare across the entire patient journey, Health Industry HubTM is the only one-stop-hub bringing the diversity of Pharma, MedTech, Diagnostics & Biotech sectors together to inspire meaningful change.
In reimagining healthcare across the entire patient journey, Health Industry HubTM is the only one-stop-hub bringing the diversity of Pharma, MedTech, Diagnostics & Biotech sectors together to inspire meaningful change.
The content on Health Industry Hub is copyright protected and should only be accessed under individual user licenses. To subscribe, please click here and visit T&Cs here.
Human Resources

Medtronic HR Director deep dives into shifting workplaces and leadership trends: International HR Day
People & Culture: Coinciding with International HR Day, Karen Newell, Senior HR Director for ANZ at Medtronic, joined Health Industry […]
MoreNews & Trends - MedTech & Diagnostics
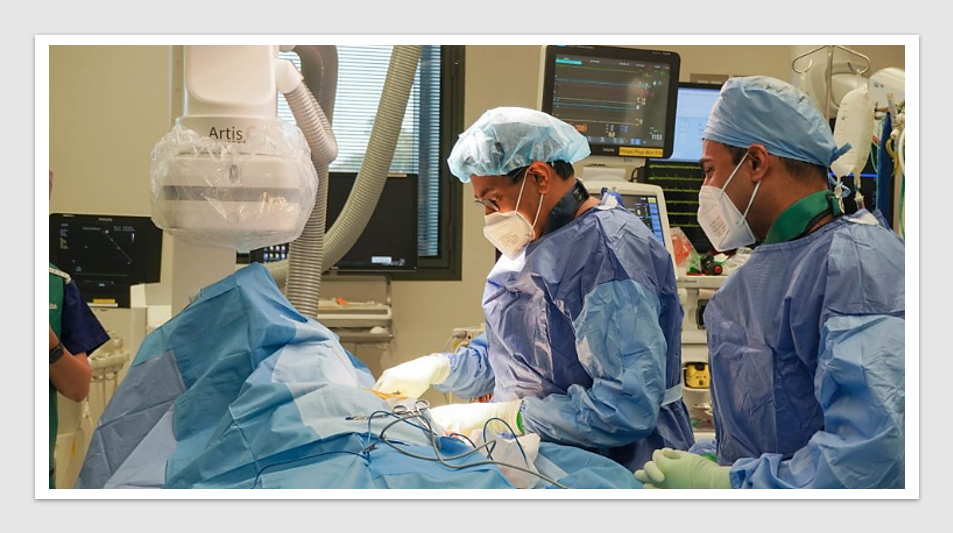
Aussie medtech reveals new data on next-gen pulsed field ablation system for atrial fibrillation
MedTech & Diagnostics News: An Australian-based medtech company announced promising results from the first-in-human study for its pulsed field ablation […]
MoreNews & Trends - MedTech & Diagnostics

Federal budget delivers ‘mixed news’ for radiologists
MedTech & Diagnostics News: The Royal College of Australian and New Zealand Radiologists (RANZCR) and the Interventional Radiology Society of […]
MoreNews & Trends - Pharmaceuticals

AstraZeneca, BMS and Noxopharm address findings of inquiry report into rare cancers
Pharma News: The Senate Community Affairs References Committee has released its inquiry report into Equitable access to diagnosis and treatment […]
More