News & Trends - Pharmaceuticals
Oxford/AstraZeneca COVID-19 vaccine trial paused due to suspected adverse reaction

Pharma News: A large, phase 3 study trial of a COVID-19 vaccine being developed by AstraZeneca and the University of Oxford has been put on hold due to a suspected serious adverse reaction in a participant in the United Kingdom.
In the statement from AstraZeneca Australia, the company spokesperson said “As part of the ongoing randomised, controlled global trials of the Oxford coronavirus vaccine, our standard review process was triggered and we voluntarily paused vaccination to allow review of safety data by an independent committee. This is a routine action which has to happen whenever there is a potentially unexplained illness in one of the trials, while it is investigated, ensuring we maintain the integrity of the trials. In large trials illnesses will happen by chance but must be independently reviewed to check this carefully. We are working to expedite the review of the single event to minimise any potential impact on the trial timeline. We are committed to the safety of our participants and the highest standards of conduct in our trials.”
On Monday 7 September 2020, Prime Minister Scott Morrison and Health Minister Greg Hunt announced onshore manufacturing agreements for Oxford/AstraZeneca and UQ/CSL COVID-19 vaccines with early access to the vaccines in January and February 2021.
The Oxford/AstraZeneca vaccine, known as AZD1222, uses an adenovirus that carries a gene for one of the proteins in SARS-CoV-2, the virus that causes Covid-19. The adenovirus is designed to induce the immune system to generate a protective response against SARS-2. The platform has not been used in an approved vaccine, but has been tested in experimental vaccines against other viruses, including the Ebola virus.
At this time it is not clear what the adverse reaction was, how long the hold will last, or how much it could delay the vaccine’s development.
Human Resources

Medtronic HR Director deep dives into shifting workplaces and leadership trends: International HR Day
People & Culture: Coinciding with International HR Day, Karen Newell, Senior HR Director for ANZ at Medtronic, joined Health Industry […]
MoreNews & Trends - MedTech & Diagnostics
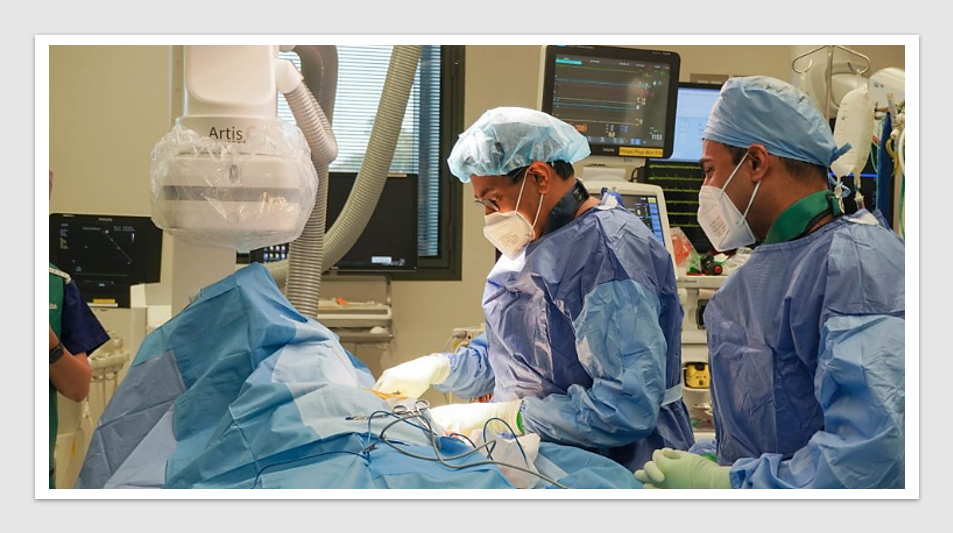
Aussie medtech reveals new data on next-gen pulsed field ablation system for atrial fibrillation
MedTech & Diagnostics News: An Australian-based medtech company announced promising results from the first-in-human study for its pulsed field ablation […]
MoreNews & Trends - MedTech & Diagnostics

Federal budget delivers ‘mixed news’ for radiologists
MedTech & Diagnostics News: The Royal College of Australian and New Zealand Radiologists (RANZCR) and the Interventional Radiology Society of […]
MoreNews & Trends - Pharmaceuticals

AstraZeneca, BMS and Noxopharm address findings of inquiry report into rare cancers
Pharma News: The Senate Community Affairs References Committee has released its inquiry report into Equitable access to diagnosis and treatment […]
More