News & Trends - Pharmaceuticals
TGA approves Pfizer-BioNTech COVID-19 vaccine

Pharma News: Pfizer Australia and BioNTech today announced that the Therapeutic Goods Administration (TGA) in Australia has granted Provisional Approval for their COVID-19 mRNA vaccine COMIRNATY™.
The distribution of the vaccine in Australia will be prioritised by the Department of Health according to the populations identified in guidance from the ATAGI (Australian Technical Advisory Group on Immunisation) COVID-19 Working Group.
“Today’s Provisional Approval in Australia marks an historic moment in the fight against COVID-19. It further affirms Pfizer’s commitment to deliver on its promise to safely bring to Australians a high quality vaccine against this virus,” said Anne Harris, Pfizer Australia and New Zealand Managing Director.
“We commend the TGA for its careful assessment of COMIRNATY™. We thank both the Commonwealth Government and the Department of Health for their strong partnership to bring our vaccine to Australians. We are proud to be part of this breakthrough which was made possible through unparalleled collaboration between companies, governments, regulators, public health bodies, and the academic and scientific communities coming together urgently to find solutions to the pandemic”, Ms Harris said.
“It is encouraging to see that our mRNA vaccine is now approved in Australia. The number of countries authorising the use of our vaccine is steadily increasing. This is important in order to support addressing this pandemic,” said Sean Marett, Chief Business Officer and Chief Commercial Officer at BioNTech.
“Together with our partner Pfizer, we are looking forward to shipping the vaccines to Australia”, Mr Marrett said.
Prime Minister Scott Morrison said the TGA approval was an important step in the fight against COVID-19.
“I welcome the TGA’s approval of the Pfizer vaccine, with our own Australian experts finding it is safe, effective and of a high standard,” the Prime Minister said.
“Australians should take confidence in the thorough and careful approach taken by our world-class safety regulator. Our priority has always been to keep Australians safe and protect lives and livelihoods. Today’s approval is another big step forward for our community, particularly in the protection of our most vulnerable people.”
The TGA’s decision is based on a rolling submission including data from the Phase 3 clinical study, which demonstrated a vaccine efficacy rate of 95% (p<0.0001) in participants without prior SARS-CoV-2 infection (first primary objective), and also in participants with and without prior SARS-CoV-2 infection (second primary objective), in each case measured from 7 days after the second dose.
The first primary objective analysis is based on 170 cases of COVID-19, as specified in the study protocol. Efficacy was consistent across age, gender, race and ethnicity demographics, with an observed efficacy in adults age 65 and over, of more than 94%. In the trial, COMIRNATY™. was generally well tolerated with no safety concerns reported by the Data Monitoring Committee to date.
Minister for Health Greg Hunt said the-world class regulators at the TGA have been working tirelessly to introduce a safe and effective COVID-19 vaccine in Australia.
“The TGA’s processes are I believe the best in the world and we have ensured that they are thorough. The TGA has placed safety above all else. Australia’s high bar has been met; the vaccine has been approved as effective in stopping severe disease. I thank all those involved in the development and assessment of this COVID-19 vaccine, including the researchers, Pfizer, BioNTech and the medical experts at the TGA who have worked around the clock and over Christmas. This approval and the upcoming roll out of the vaccine will play an important part in our ability to manage the pandemic in 2021,”Minister Hunt said.
Pfizer and BioNTech previously announced an agreement with the Australian government on 5 November 2020 to supply 10 million doses of the mRNA-based vaccine COMIRNATY™ once approved. Dose deliveries will occur throughout 2021 in accordance with the terms of the supply agreement.
The vaccine has now been granted a conditional marketing authorization, emergency use authorisation, temporary authorisation or provisional approval in a total of more than 50 countries.
News & Trends - Biotechnology

CSL reshapes R&D while bracing for U.S. tariffs
Australia’s largest biotech company CSL is streamlining its R&D operations to enhance efficiency amidst a rapidly evolving global landscape. The […]
MoreNews & Trends - MedTech & Diagnostics
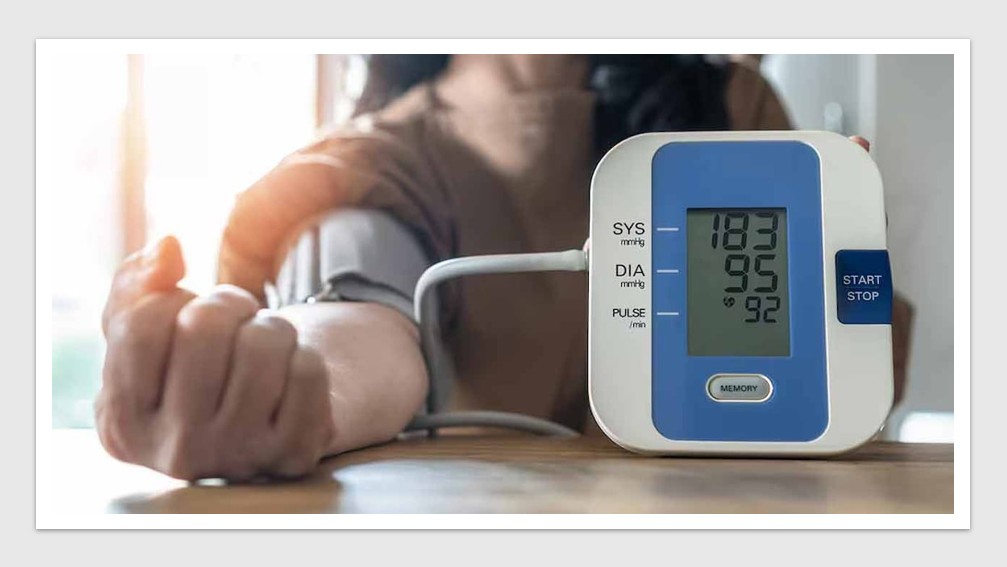
Australia joins Medtronic trial in fight against resistant hypertension
Medtronic has launched an international clinical trial across Australia, the United States, and Europe to evaluate the feasibility of multi-organ […]
MoreNews & Trends - MedTech & Diagnostics

Medibank launches pharmacogenetic testing while government stalls on insurance discrimination ban
Medibank has become the first Australian health insurer to pay towards pharmacogenetic testing (PGx) for eligible customers on Extras cover. […]
MoreNews & Trends - Pharmaceuticals

Global pledge shifts visibility and action for patients with advanced breast cancer
Three breast cancer organisations have united internationally to demand that people living with metastatic breast cancer (MBC) are no longer […]
More