News & Trends - Pharmaceuticals
Sanofi and GSK initiate clinical trial of vaccine candidate

Pharma News: Sanofi and GSK initiated the Phase 1/2 clinical trial for their adjuvanted COVID-19 vaccine. The vaccine candidate, developed in partnership by Sanofi and GSK, uses the same recombinant protein-based technology as one of Sanofi’s seasonal influenza vaccines with GSK’s established pandemic adjuvant technology.
The Phase 1/2 clinical trial is a randomised, double blind and placebo-controlled trial designed to evaluate the safety, reactogenicity (tolerability) and immunogenicity (immune response) of the COVID-19 vaccine candidate. A total of 440 healthy adults are being enrolled in the trial across 11 investigational sites in the United States.
The companies anticipate first results early December 2020 which will support the initiation of a Phase 3 trial in December 2020. If data are sufficient for licensure application, the plan is to request regulatory approval in the first half of 2021.
Sanofi is leading the clinical development and registration of the COVID-19 vaccine. Preclinical data showed an acceptable reactogenicity profile and data based on two injections of the adjuvanted recombinant vaccine showed high levels of neutralizing antibodies that are comparable to levels in humans who recovered from the COVID-19 infection. Pre-clinical results will be published later this year. In parallel, Sanofi and GSK are scaling up manufacturing of the antigen and adjuvant with the target of producing up to one billion doses in 2021.
“Sanofi and GSK bring proven science and technology to the fight against the global COVID-19 pandemic, with the shared objective of delivering a safe and effective vaccine,” said Thomas Triomphe, Executive Vice President and Global Head of Sanofi Pasteur. “The initiation of our clinical study is an important step and brings us closer to a potential vaccine which could help defeat COVID-19. Our dedicated teams and partner continue to work around the clock as we aim to deliver the first results in early December. Positive data will enable a prompt start of the pivotal phase 3 trial by the end of this year.”
Roger Connor, President of GSK Vaccines added, “Moving this vaccine candidate into clinical development is an important moment in the progress towards addressing the global pandemic we are all facing. This builds on the confidence shown by governments already in the potential of this protein-based adjuvanted vaccine candidate, which utilizes established technology from both companies, and can be produced at scale by two of the leading vaccine manufacturers globally. We now look forward to the data from the study, and if positive, beginning Phase 3 by the end of the year.”
News & Trends - Biotechnology

CSL reshapes R&D while bracing for U.S. tariffs
Australia’s largest biotech company CSL is streamlining its R&D operations to enhance efficiency amidst a rapidly evolving global landscape. The […]
MoreNews & Trends - MedTech & Diagnostics
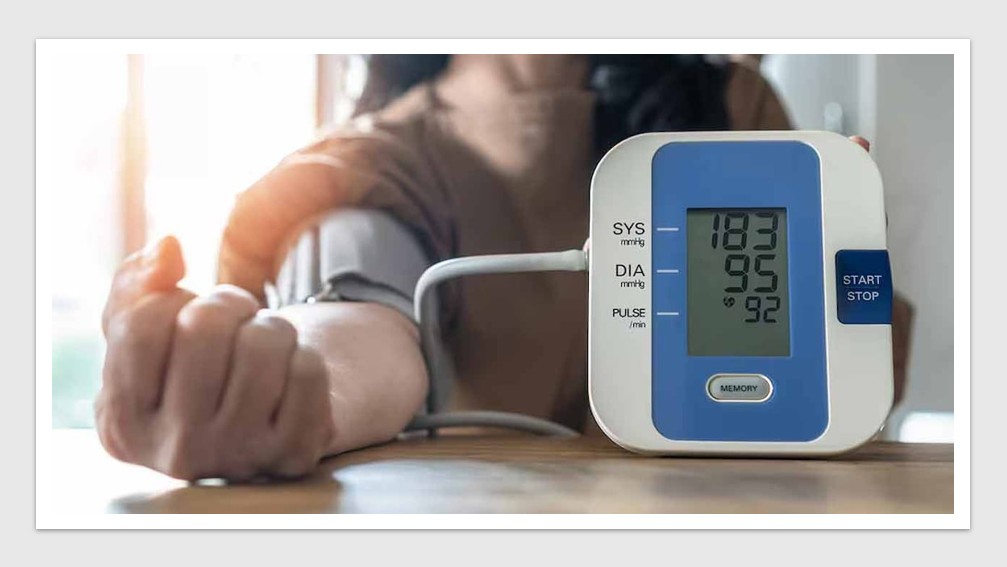
Australia joins Medtronic trial in fight against resistant hypertension
Medtronic has launched an international clinical trial across Australia, the United States, and Europe to evaluate the feasibility of multi-organ […]
MoreNews & Trends - MedTech & Diagnostics

Medibank launches pharmacogenetic testing while government stalls on insurance discrimination ban
Medibank has become the first Australian health insurer to pay towards pharmacogenetic testing (PGx) for eligible customers on Extras cover. […]
MoreNews & Trends - Pharmaceuticals

Global pledge shifts visibility and action for patients with advanced breast cancer
Three breast cancer organisations have united internationally to demand that people living with metastatic breast cancer (MBC) are no longer […]
More