News & Trends - Pharmaceuticals
Johnson & Johnson pauses COVID-19 vaccine trial

Pharma News: Johnson & Johnson confirmed it has temporarily paused its COVID-19 vaccine clinical trials due to an unexplained illness in a study participant.
While the company did not elaborate on the illness, the pause follows a similar action by British-Swedish pharmaceutical company AstraZeneca, who paused their trial in September, however their trials have since resumed.
Jeremy Nicholson, Pro-Vice Chancellor of Health Sciences at Murdoch University and Director of the Australian National Phenome Centre, said “It is not unusual to suspend a clinical trial if there is an adverse-reaction reported during testing. This is all part of checking that the vaccine (or drug) is safe. People can fall ill in clinical trials by chance and it may have nothing to do with the vaccine itself, this is especially likely in a large trial. Each case has to be investigated thoroughly to evaluate the cause and likelihood of it being trial related.
“There was also a recent pause in the AstraZeneca SARS CoV-19 vaccine trial because of reported neurological side-effects. The Johnson &Johnson participant has not yet been identified, but it would be more worrying if it were also a neurological effect. Vaccines can sometimes cause the same virus-related problems that they are meant to prevent. Worryingly, there are now multiple reports of neuropathological effects of COVID-19 of varying presentation and severity, so if it were to be a neuro side problem that would need special attention.
“It is far too early to tell if there is a generic safety problem with respect to neurological (or other organ systems) effects of any of the new SARS CoV-19 vaccines – that’s what the testing is for. This is the reason that vaccine trials take a long time, to establish the safety of the treatment when given at large scale. Suspending a study due to possible side effects (connected or unconnected to the treatment) is part of normal good practise in vaccine development and shows that the study is being done properly.
“There can be no rushed release of a drug, vaccine or antibody cocktail when the safety of millions of people is at stake – no matter what the political pressure. Hopefully, it will be shown that this is an effect unrelated to treatment so that the study can recommence as soon as possible. The world needs a vaccine because COVID-19 is not going away on its own.”
Professor Raina MacIntyre, Head of the Biosecurity Program at the Kirby Institute at the University of NSW, said “The pausing of the J&J vaccine trial tells us the strict safety monitoring is occurring, and this should reassure people that the correct process is being followed. The J&J vaccine, like the AstraZeneca vaccine and other candidates, use an adenovirus vector – a harmless virus that does not multiply in humans – to deliver the part of the SARS-CoV-2 virus protein that elicits antibodies. No licensed vaccines in the past have used an adenovirus vector. We do not yet know what illness the trial participant suffered, but we can assume it was a serious enough illness to pause the trial.
The key questions are:
- Is this caused by the vaccine, or is it incidental and would have happened to this person anyway?
- If caused by the vaccine, which component of the vaccine caused it? It could be the vector or the protein from SARS-CoV-2.
“We determine if a vaccine adverse event is causal or coincidental by looking at the rates of the event in vaccinated and unvaccinated people in the clinical trial. If there is no difference in the two groups, that is reassuring. If, however, the rate of the adverse event is statistically significantly higher in the vaccinated group, that indicates it is likely caused by the vaccine.
All trials have safety committees that carefully evaluate the evidence and decide on whether the vaccine caused the event. In some cases, vaccine adverse events are so rare that they will not be detected in standard clinical trials. This happened with the dengue vaccine, which showed no safety issue in clinical trials, but antibody-dependent enhancement (ADE) occurred when it was used in a large scale vaccination program in the Philippines, with far higher numbers of people vaccinated than in the clinical trials. The same occurred with the very first rotavirus vaccine – a very rare side effect, a bowel disorder in children, was not seen in the clinical trials, but became apparent when the US began vaccinating infants with it in the 1990s. That vaccine was withdrawn and the subsequent, newer rotavirus vaccines had to undergo massive scale clinical trials (>60,000 people) to prove they did not cause that same problem. So a clinical trial is not the end of the story.
“This is why we do safety monitoring post-licensure of vaccines. Our old vaccines are tried and tested in this way, and extremely safe. A new vaccine has to be rigorously tested to ensure safety.
“As more and more vaccines go through phase 3 trials, we will find some are more effective, and some are safer. Australia should diversify our options for vaccines to ensure we have enough options to choose the best ones when the time comes. If we focus only on one or two vaccines, and they end up being non-starters or have serious side effects, we could be left out in the cold. It is best to choose several different vaccine technologies – MRNA-based, vectored, subunit etc – as each technology will have unique features and may also be associated with safety,” concluded Professor MacIntyre.
News & Trends - Biotechnology

CSL reshapes R&D while bracing for U.S. tariffs
Australia’s largest biotech company CSL is streamlining its R&D operations to enhance efficiency amidst a rapidly evolving global landscape. The […]
MoreNews & Trends - MedTech & Diagnostics
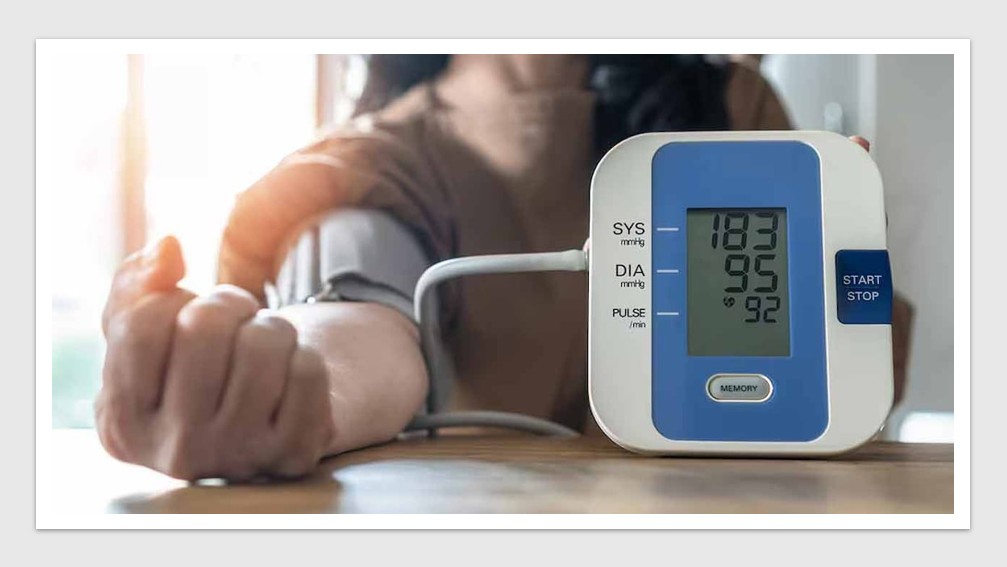
Australia joins Medtronic trial in fight against resistant hypertension
Medtronic has launched an international clinical trial across Australia, the United States, and Europe to evaluate the feasibility of multi-organ […]
MoreNews & Trends - MedTech & Diagnostics

Medibank launches pharmacogenetic testing while government stalls on insurance discrimination ban
Medibank has become the first Australian health insurer to pay towards pharmacogenetic testing (PGx) for eligible customers on Extras cover. […]
MoreNews & Trends - Pharmaceuticals

Global pledge shifts visibility and action for patients with advanced breast cancer
Three breast cancer organisations have united internationally to demand that people living with metastatic breast cancer (MBC) are no longer […]
More