News & Trends - MedTech & Diagnostics
Robotic surgery: Finding evidence of benefit is challenging
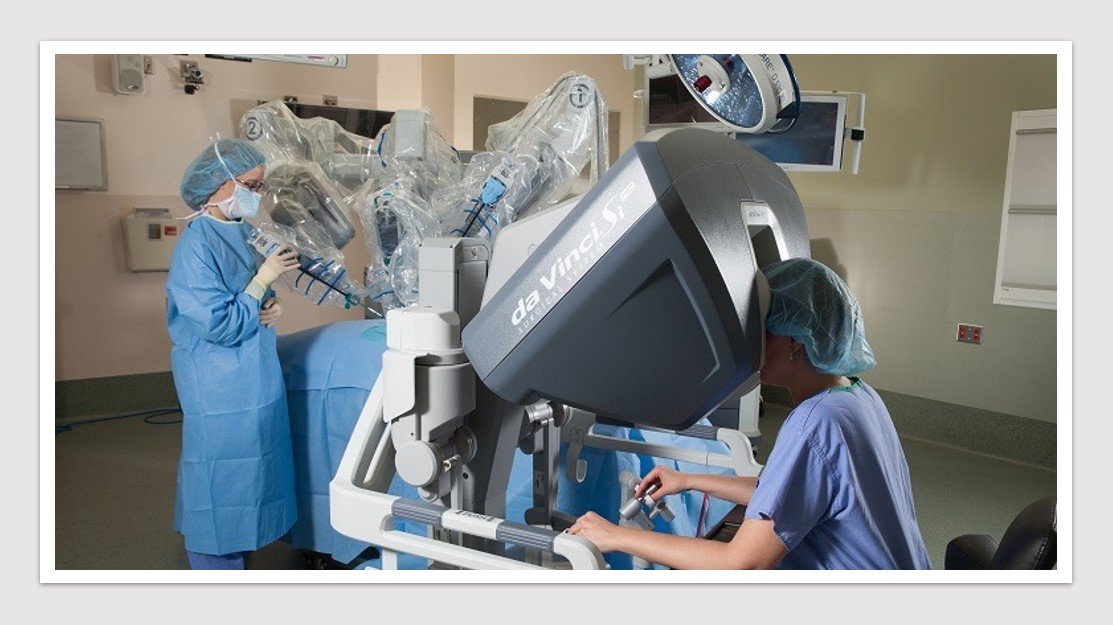
MedTech News: Finding high-quality evidence supporting the use of robotic surgery remains challenging with surgeons themselves the biggest hurdle, according to the authors of a Perspective published in the Medical Journal of Australia.
“Approximately 1.2 million robotic procedures had been performed worldwide as of December 2020, most of which were robotic-assisted radical prostatectomies (RARPs),” wrote the authors, led by Dr Wei Shen Tan, Honorary Clinical Lecturer and Urologic Oncology Fellow at the University College London.
“By contrast, many colorectal and gynaecological procedures have remained within the remit of laparoscopic or traditional open surgery. Opponents of robotic surgery often cite the lack of evidence to support its use and highlight the high healthcare cost.”
In Australia, the cost of the da Vinci Xi platform (manufactured by Intuitive Surgical, distributed by Device Technologies) is an estimated $3.9 million in addition to consumable costs of $1,848 per operation and a service cost of $621,245 for a 3‐year contract. Intuitive has enjoyed a monopoly, although the introduction of new robotic systems into the market has the potential to alter the health economic landscape. As competition from newer robotic systems drives cost down, it is conceivable that robust evidence will be important in overcoming barriers to adoption.
Advances in robotics surgery to pave the way for wider access, says Professor Tony Costello
Perceived benefits of robotic surgery, despite little or no evidence of benefit, are “often enough for both patients and surgeons to choose a new technology,” Tan and colleagues wrote.
One challenge for conducting research is that key opinion leaders adopt the technology early and then drive expansion and use before safety and efficacy data are collected. Once the technology is in use, there is then little motivation to conduct clinical trials.
“RARP, first described in 2002, is now the standard of care in most developed countries and was widely adopted despite little evidence for benefit previously,” wrote Tan and colleagues. “It was not until 2016, that the first well designed RCT reported outcomes.”
The authors detailed randomised control trials of robotic surgery in prostate cancer and wrote that “evidence purporting to show a benefit for robotic surgery has not been forthcoming to other surgical procedures” either.
“In the case of radical hysterectomy for early-stage cervical cancer, a robotic-assisted procedure may result in inferior oncological outcomes,” Tan and colleagues wrote, citing an RCT (Ramirez et al 2018, Lewicki et al 2021) of 631 patients which reported that the minimally invasive arm (including robotic-assisted surgery) resulted in lower disease-free survival and overall survival.
“Surgeons remain the main obstacle to the success of surgical randomised trials,” they wrote.
“In our pursuit of high-quality evidence, we owe it to our patients to set aside personal views, acknowledge that limited evidence is available in certain areas of surgical practice, and support surgical trial recruitment.
“New technologies should be evaluated in a prompt manner before widespread dissemination. Evaluating new technologies in an evidence-based approach in collaborative centralised health networks group within surgical technology hubs may aid rapid patient recruitment, particularly in complex and uncommon surgical procedures,” they concluded.
“This could then enable prompt trial completion before adoption of such technologies and before they are entrenched as standard of care.”
 In reimagining healthcare across the entire patient journey, Health Industry HubTM is the ONLY one-stop-hub bringing the diversity of Pharma, MedTech, Diagnostics & Biotech sectors together to inspire meaningful change.
In reimagining healthcare across the entire patient journey, Health Industry HubTM is the ONLY one-stop-hub bringing the diversity of Pharma, MedTech, Diagnostics & Biotech sectors together to inspire meaningful change.
The exclusive leadership and influencer podcasts and vodcasts add huge value to our breaking news coverage. The content on Health Industry Hub is copyright protected and should only be accessed under individual user licenses. Please click here to subscribe and visit T&Cs here.
News & Trends - Biotechnology

Aussie biotech secures agreement with UQ and USyd to advance cardiovascular diseases research
Biotech News: Australian biotechnology company, Cartherics, developing immune cell therapies for the treatment of cancer, has entered into a Technology […]
MoreNews & Trends - MedTech & Diagnostics

NSW Health Secretary urges more focus on patient experience, drawing from her own heart valve disease journey
In a heart-warming event that united patients, their loved ones, representatives from patient organisations and employees, Edwards Lifesciences hosted its […]
MoreHuman Resources

‘To be an inclusive society, we need an embedded national strategy to combat systemic racism’, says Commissioner Sivaraman
Race Discrimination Commissioner Giridharan Sivaraman has welcomed the release of the Multicultural Framework Review by the Federal Government, calling it […]
MoreNews & Trends - Pharmaceuticals

Shingles vaccine lowers risk of dementia
Pharma News: A recombinant shingles vaccine which was added to Australia’s National Immunisation Program (NIP) in November last year, is […]
More