News & Trends - MedTech & Diagnostics
Reduced TGA charges for medical devices listed on the prostheses list

MedTech News: In response to the suspension of elective surgery due to COVID-19 and the significant impact this has had on parts of the medical devices industry, the Government has agreed to provide a 50% reduction in annual charges for certain medical devices.
The 50% reduction in annual charges will be prescribed for the 2020-21 financial year for medical devices included in the Australian Register of Therapeutic Goods (ARTG) as Class IIa, IIb, III and AIMD and which are listed prostheses as defined in the Private Health Insurance (Prostheses) Rules (No.1) 2020, as in force on 8 April 2020.
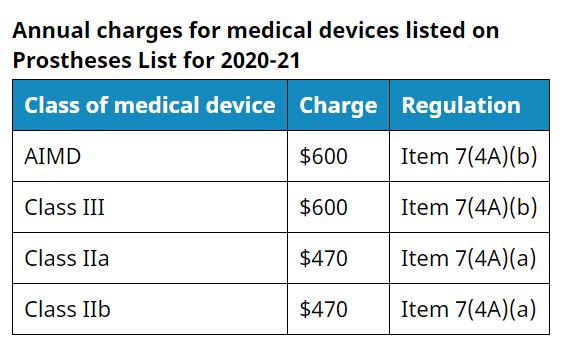
These charges are in the Therapeutic Goods (Charges) Regulations 2018
No action is required by eligible sponsors as the Therapeutic Goods Administration (TGA) will use the published Prostheses List (PL) as in force on 8 April 2020 with reference to the ARTG inclusion number to identify those devices which will be subject to the reduced charges. If the ARTG inclusion number for a device on the PL as in force on 8 April 2020 is not correct, contact the TGA by 31 July 2020.
The reduced annual charge would also apply to an ARTG entry where multiple products are included in the entry and only some products covered by the entry are listed on the PL.
Sponsors will receive an invoice in August for the 2020-21 annual charges, which will automatically include the reduced charges for such devices.
The complete TGA fees and charges schedule is available on the TGA website.
Digital & Innovation

Medical drone to reduce health equity gaps in rural and remote Australia
A specialised medical drone which increases accessibility to essential health services such as pathology, medicines, and telehealth services in rural […]
MoreNews & Trends - Pharmaceuticals

We’ve spent more on healthcare, but it’s been worth it
Healthcare expenditure is surging, with Australia now allocating approximately one-tenth of its budget to this sector. This financial uptick prompts […]
MoreNews & Trends - Pharmaceuticals

New partnership to raise the bar in precision oncology in Queensland
Pharma News: The Australian Translational Genomics Centre (ATGC) is teaming up with non-profit research organisation Omico and the PrOSPeCT program […]
MoreNews & Trends - Biotechnology

AusBiotech appoints new CEO: Former Sanofi corporate affairs and sustainability leader takes the helm
Biotech News: AusBiotech, the nation’s leading industry body for the biotech sector, has named former leader at Sanofi, Rebekah Cassidy, […]
More