News & Trends - MedTech & Diagnostics
Legal supply of COVID-19 diagnostic tests
MedTech News: To support efforts in managing the COVID-19 emergency, the Therapeutic Goods Administration (TGA) is working with suppliers to ensure that Australia has laboratory and point of care tests (POCT) available that are able to accurately detect COVID-19 infections.
There are two pathways to legally supply these types of tests.
- Undergoing an expedited TGA assessment of the COVID-19 test for inclusion on the Australian Register of Therapeutic Goods (ARTG). Once approved for inclusion on the ARTG, the COVID-19 test can be legally supplied in Australia.
- Supply of COVID-19 tests under the new Therapeutic Goods (Medical Devices Accredited Pathology Laboratories) (COVID-19 Emergency) Exemption 2020. This exemption permits importation, manufacture and supply of COVID-19 tests (that have not undergone TGA assessment) to accredited pathology laboratories.
Customer engagement during COVID-19. Health Industry Hub combines expertise in audience insights, delivering digital health content and measuring engagement with 20+ years industry experience. Created by industry for industry. Contact us.
The TGA has now approved the following tests for inclusion on the Australian Register of Therapeutic Goods (ARTG).
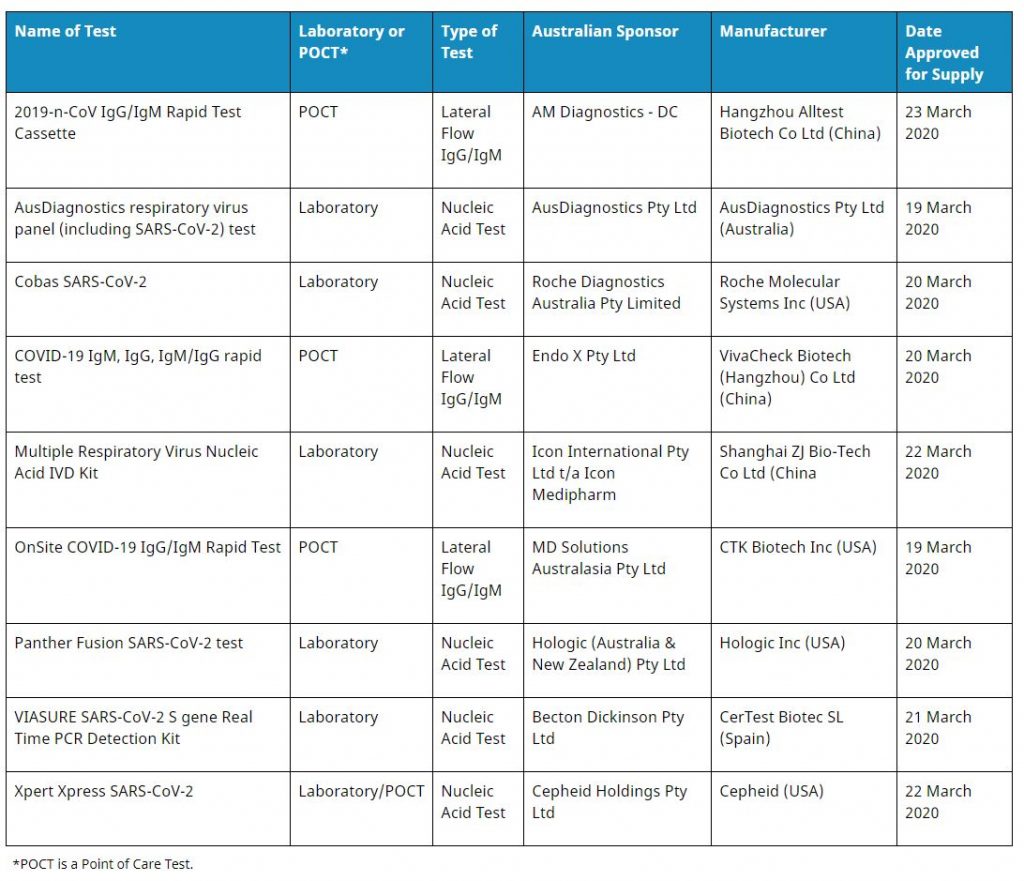
Expedited TGA assessment of COVID-19 diagnostic tests
COVID-19 is an emerging viral infectious disease. There is limited information available regarding the disease profile and the ability of available tests to accurately detect COVID-19 infections.
The TGA is currently undertaking an expedited assessment process based on the information and performance data currently available at the time of application for inclusion on the ARTG. All COVID-19 tests that are included on the ARTG based on this expedited assessment process are subject to additional non-standard conditions, which makes it easier for the TGA to perform additional post market assessments as experience and knowledge around COVID-19 diagnostic testing grows.
The conditions require that additional evidence to support the ongoing safety and performance of the devices be provided to the TGA within 12 months of approval.
Register FREE and join 20,000+ industry professionals who receive the latest industry news, innovations and insights from Health Industry Hub; the ONLY one-stop-hub connecting Australia’s Pharma, MedTech and Biotech industry professionals.
Exemption for supply of COVID-19 tests to accredited pathology laboratories
A new emergency exemption, Therapeutic Goods (Medical Devices Accredited Pathology Laboratories) (COVID-19 Emergency) Exemption 2020, has been made to allow rapid supply of COVID-19 diagnostic tests to all Australian accredited pathology laboratories.
In practice this emergency exemption allows COVID-19 diagnostic tests to be immediately supplied to accredited pathology laboratories approved under the Health Insurance Act 1973, while the TGA continues to expedite the regulatory assessment process for these devices.
The exemption does not allow for general supply of rapid tests, including serological rapid tests intended for use at the point of care (POCT), other than to the accredited pathology laboratories specified in the exemption. Although, point of care tests (POCT) that have been included on the ARTG will be available for broader supply within Australia.
News & Trends - Biotechnology

Aussie biotech secures agreement with UQ and USyd to advance cardiovascular diseases research
Biotech News: Australian biotechnology company, Cartherics, developing immune cell therapies for the treatment of cancer, has entered into a Technology […]
MoreNews & Trends - MedTech & Diagnostics

NSW Health Secretary urges more focus on patient experience, drawing from her own heart valve disease journey
In a heart-warming event that united patients, their loved ones, representatives from patient organisations and employees, Edwards Lifesciences hosted its […]
MoreHuman Resources

‘To be an inclusive society, we need an embedded national strategy to combat systemic racism’, says Commissioner Sivaraman
Race Discrimination Commissioner Giridharan Sivaraman has welcomed the release of the Multicultural Framework Review by the Federal Government, calling it […]
MoreNews & Trends - Pharmaceuticals

Shingles vaccine lowers risk of dementia
Pharma News: A recombinant shingles vaccine which was added to Australia’s National Immunisation Program (NIP) in November last year, is […]
More