News & Trends - MedTech & Diagnostics
First commercial diagnostic to enable expedited coronavirus testing to meet urgent medical needs
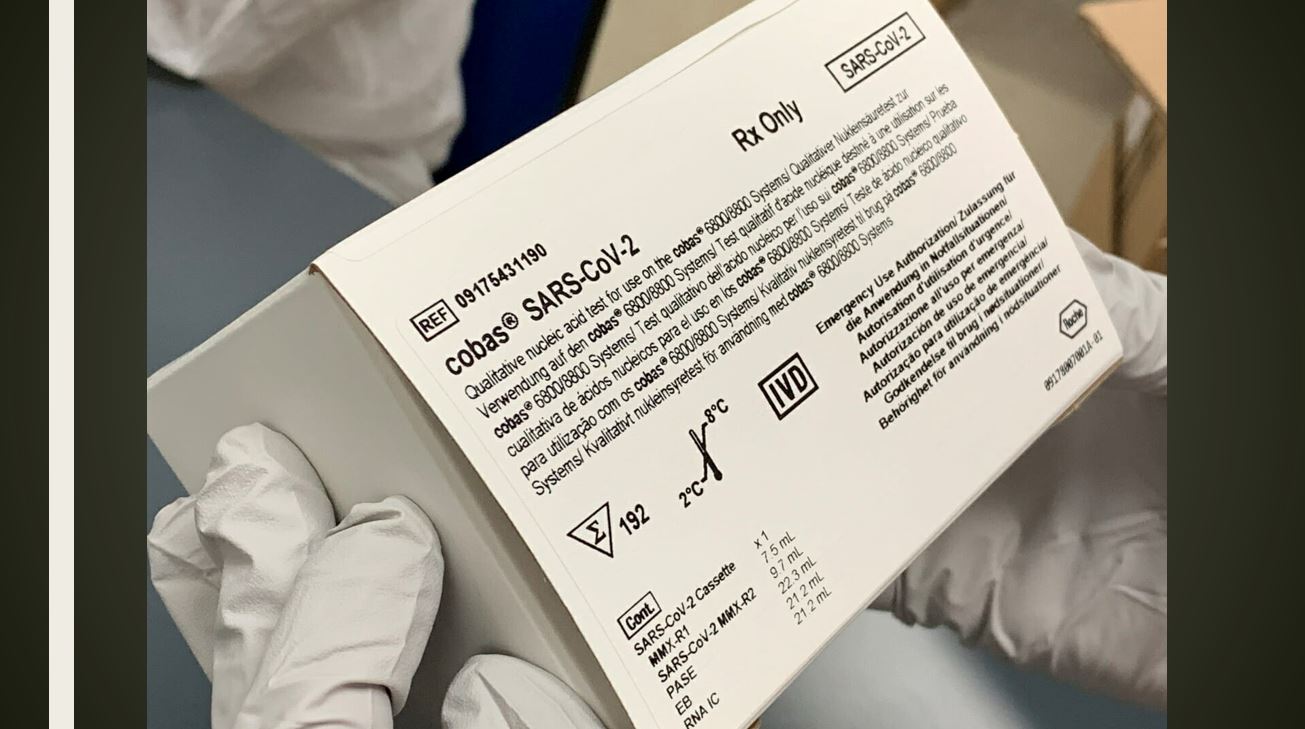
MedTech News: Roche’s cobas SARS-CoV-2 Test to detect novel coronavirus receives FDA Emergency Use Authorisation and is available in markets accepting the CE mark.
It is intended for the qualitative detection of SARS-CoV-2, the virus that causes COVID-19 disease, in nasopharyngeal and oropharyngeal swab samples from patients who meet COVID-19 clinical and/or epidemiological criteria for testing. Hospitals and reference laboratories can run the test on Roche’s fully automated cobas 6800 and cobas 8800 Systems, which are widely available around the world.
The CE-IVD test is available in markets accepting the CE mark for patients with signs and symptoms of COVID-19 disease and living in affected areas where the SARS-CoV-2 virus is known to be present.
Register FREE and join 20,000+ industry professionals who receive the latest industry news, innovations and insights from Health Industry Hub; the only one-stop-hub connecting Australia’s Pharma, MedTech and Biotech industry professionals and its key stakeholders.
The widely available Roche’s cobas 6800/8800 Systems, which are used to perform the cobas SARS-CoV-2 Test, provide test results in three and half hours and offer improved operating efficiency, flexibility, and fastest time-to-results with the highest throughput providing up to 96 results in about three hours and a total of 384 results for the cobas 6800 System and 960 results for the cobas 8800 System in 8 hours. The test can be run simultaneously with other assays provided by Roche for use on the cobas 6800/8800 Systems.
“Providing quality, high-volume testing capabilities will allow us to respond effectively to what the World Health Organisation has characterised as a pandemic. It is important to quickly and reliably detect whether a patient is infected with SARS-CoV-2,” said Thomas Schinecker, CEO of Roche Diagnostics. “Over the last weeks, our emergency response teams have been working hard to bring this test to the patients. CE-mark certification and the FDA’s granting of EUA supports our commitment to give more patients access to reliable diagnostics which are crucial to combat this serious disease.”
These instruments are available in a number of laboratories across all Australian states. Roche expects the first shipment of these new cobas SARS-CoV-2 tests to arrive in Australia on Wednesday 18 March. With these two tests available, it is anticipated that approximately 100,000 test kits will be delivered to Australia this week.
Roche will be reordering both test kits frequently, based on Australian patient needs. We will continue to have both tests available in Australia, to ensure maximum coverage and patient reach. We are continuing to work with Australian laboratories and the Federal Government to ensure locations of greatest need are prioritised and people at the highest risk of COVID-19 have access to these tests.
Enhance corporate branding and boost thought leadership to attract and retain quality employees. How does your company culture and digital footprint measure up in the Australian Medical Devices industry? Get the benchmark report by contacting us.
You may also like Roche diabetes monitoring products to be sold in Woolworths
News & Trends - Biotechnology

AusBiotech appoints new CEO: Former Sanofi corporate affairs and sustainability leader takes the helm
Biotech News: AusBiotech, the nation’s leading industry body for the biotech sector, has named former leader at Sanofi, Rebekah Cassidy, […]
MoreNews & Trends - MedTech & Diagnostics

Federal government invests in Siemens Healthineers scanner to ‘reduce wait times’ for cancer diagnosis
MedTech & Diagnostics News: The Albanese Government is investing $12 million through the 2024–25 Budget, to purchase and install a […]
MoreNews & Trends - MedTech & Diagnostics
Cardiac device benefits face more cuts, while technical services remain secure in the short term
MedTech & Diagnostics News: Starting from July 2024, Cardiac Implantable Electronic Devices (CIED) listed on the Prescribed List (PL) will […]
MoreNews & Trends - Biotechnology

CSL’s world-first gene therapy heads for MSAC evaluation
Biotech News: CSL’s world-first gene therapy for haemophilia B is scheduled for consideration at the upcoming Medical Services Advisory Committee (MSAC) […]
More