News & Trends - MedTech & Diagnostics
Australian-first procedure for chronic pain sufferers
MedTech News: Chronic pain treatment has come a long way. While the idea of blocking the human body’s own electrical impulses to fight pain has been widely utilised in modern medicine, it is only now that precise spinal-cord stimulation is capable of providing unprecedented results.
Sydney Adventist Hospital specialist and interventional pain physician Dr Vahid Mohabbati has performed an Australian-first procedure using a pioneering device to help a patient with chronic problems.
In overseas use, the matchbox-sized implant has reportedly reduced pain by up to 80% in 80% of patients. Unlike its predecessors, it can deliver a wide range of electrical currents.
“Previously there was no variability, with only 10,000 Hertz of electrical impulse able to be delivered and nothing below,” Dr Mohabbati said.
“Now this implant has the versatility to go from 2 to 10,000 Hertz and a range of different programs of electrical impulses, expanding its use for pain caused by back or nerve problems, sciatica, diabetic neuropathy, abdominal pain, pelvic pain or cancer. No other machine can do this.”
More than three million Australians live with chronic pain, costing the health system $12 billion for treatment in 2018 according to Pain Australia.
National Pain Week – Monday 27 July to Sunday August 2 – aimed to raise awareness of its impact on people of all ages.
Dr Mohabbati’s patient was a woman in her 40s debilitated by chronic back and leg pain despite having had multiple surgeries.
“Traditionally the only treatment options might have been medication, physical therapy, psychological treatment and in some cases surgery, or patients might have been told to simply live with the pain,” he said.
Electrical devices were designed to replace pain with a tingling sensation, based on the knowledge that pain signals are transmitted via electrical impulses into the spinal cord and then into higher brain centres, which perceive the pain.
However, such treatment was not effective or tolerable for all patients.
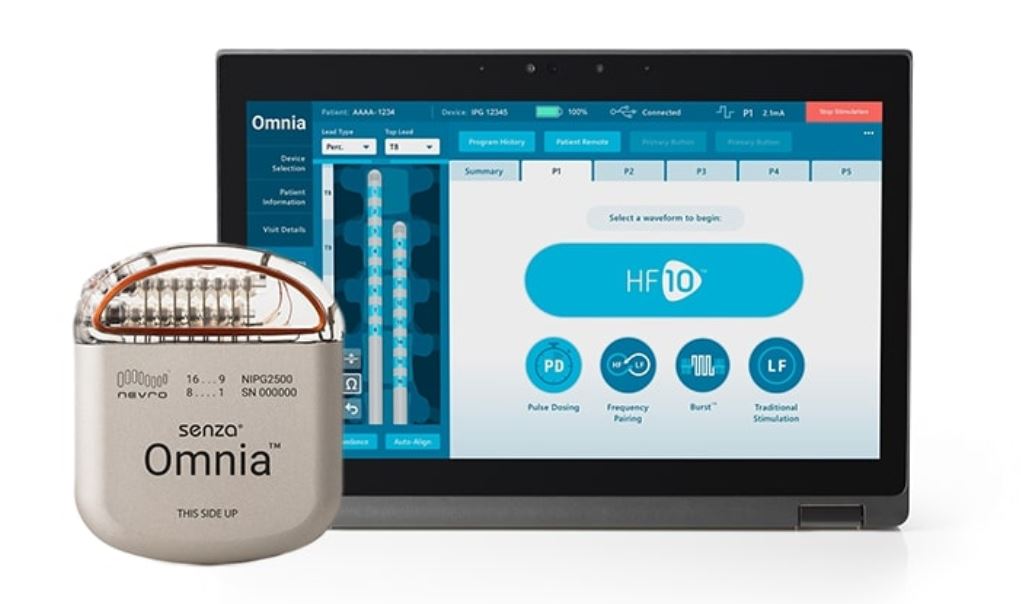
Nevro, a global medical device company, recently announced the TGA approval and July 1, 2020 Prostheses Listing for the Senza Omnia Spinal Cord Stimulation (SCS) system.
The flexibility of the new Senza Omnia device means treatment can be altered if the patient’s body gets used to impulses in a particular range.
Patients are given a week-long trial, with electrodes inserted under the skin in a day procedure and connected to an external battery.
“If pain improves by 50% then they are a candidate for a permanent implant,” Dr Mohabbati said.
The next step is a 45-minute day procedure, and the patient can then control the titanium device, monitored by a doctor.
Dr Mohabbati said it could be a vital tool in combating the growing use of prescription painkillers such as opioids, which were involved in more than 1,000 deaths in 2018 according to the Australian Bureau of Statistics.
“We have constantly been looking for improvements in non-pharmacological treatment. This is a great option, it can in fact change the lives of many,” he said.
“The TGA approval of the Omnia spinal cord stimulation system to treat chronic refractory pain will be a welcome new platform from Nevro,” said Nick Christelis, Pain Specialist Physician, Director of Pain Specialists Australia and current president of NSANZ. “I’m looking forward to using Omnia’s updated programming suite which reflects a more versatile solution to treat the changing, dynamic, and sometimes even progressive, nature of chronic pain.”
News & Trends - MedTech & Diagnostics

AI-assisted colonoscopy boosts polyp and adenoma detection
MedTech & Diagnostics News: In a standard colonoscopy, as many as one-third of colorectal polyps and adenomas can go by […]
MoreNews & Trends - Pharmaceuticals

‘Every day of delay is costing Australian lives’, says Rare Cancers Australia CEO
Pharma News: Rare Cancers Australia (RCA) has voiced its disappointment alongside the pharmaceutical industry following the Pharmaceutical Benefits Advisory Committee’s […]
MoreNews & Trends - Pharmaceuticals

Government’s silence on Senate report leaves cancer patients in limbo
Pharma News: NeuroEndocrine Cancer Australia has urged the government to respond to the Senate report on equitable access to diagnosis […]
More