News & Trends - Biotechnology
Vaxxas collaborates with USyd in development of needle-free vaccine patch
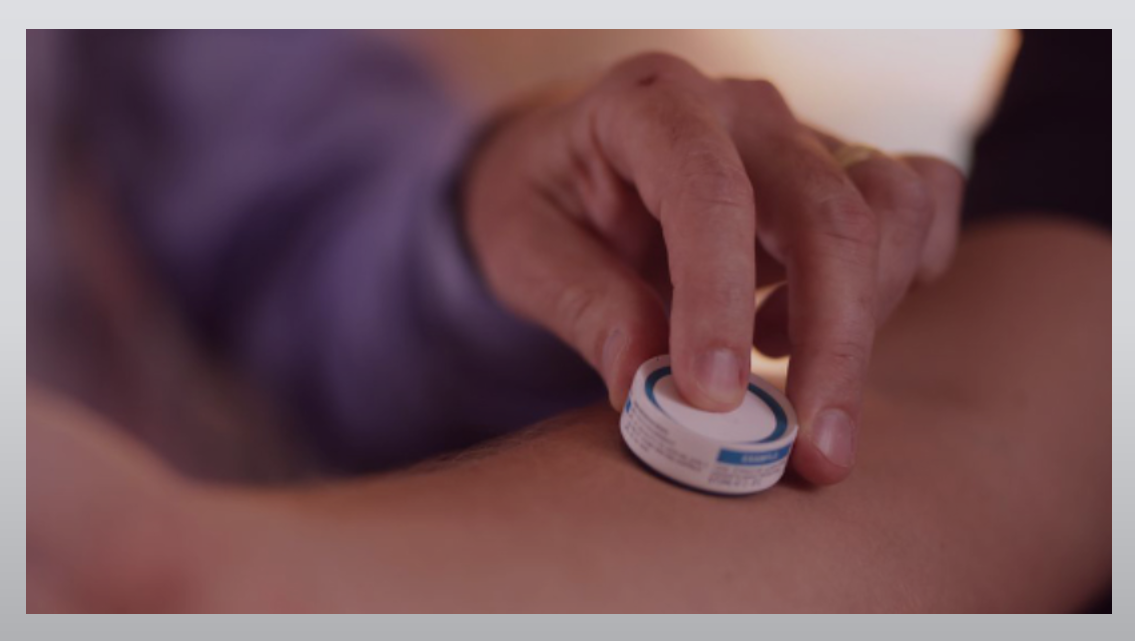
Biotech News: University of Sydney researchers have been awarded $1.12 million in funding via the Innovative Manufacturing CRC (IMCRC) to undertake independent clinical research studies to understand the potential of needle-free vaccine delivery for at-risk groups.
This grant reflects matched funding from Commonwealth Government funded IMCRC and Vaxxas, an Australian biotechnology company.
The two upcoming clinical studies are designed to evaluate the safety, feasibility, acceptability and usability of self-administration of Vaxxas’ vaccine delivery technology using an inactive substance. They will focus on older adults and healthcare professionals who are more likely to be impacted by pandemic influenza and SARS-COV-2.
Lead researcher Professor Rachel Skinner from the University of Sydney’s Faculty of Medicine and Health said the device presents potential advantages compared to vaccination using a needle and syringe.
The device is a one square-centimetre of biocompatible polymer, smaller than a postage stamp, covered in thousands of micro-projections which are invisible to the naked eye. These are coated with a vaccine formulation, with the goal of penetrating the protective outer layer of the skin to deliver the vaccine to cell layers immediately under the skin, rich in immune cells.
The device is applied to the skin using a disposable applicator that contains the product. The vaccine technology is still under development and has not yet been approved for use.
“The goal is for the device to only require a small dose of vaccine to generate the same level of immune response in the recipient,” said Professor Skinner.
“The device doesn’t require refrigeration making it easy and cheap to transport and store. It is designed to be simple to use, with the potential to be self-administered.”
Cristyn Davies, a Research Fellow in the Faculty of Medicine and Health, said the trials aim to simulate vaccination in a pandemic situation, focusing on priority groups susceptible to infection such as the elderly, and healthcare workers who are required to care for infectious patients.
“The studies will use an excipient, an inactive substance which is of the same texture as a vaccine, to test the effectiveness of the device at delivery of the substance into the skin.”
“With older populations, we are particularly interested to see how the technology works with those with aged, delicate skin. In both groups, we will test whether it’s feasible and acceptable for them to self-administer the patch, which could prove critical for many populations during a pandemic
situation.”
Professor Rachel Skinner said that the new studies build on earlier studies carried out by the team in 2019-2020 to test the usability and acceptability of the device among parents, clinicians and immunisation nurses, and assess cost-benefits on logistical parameters like supply chain, compared
to conventional immunisation. The results are yet to be published.
David Chuter, CEO and Managing Director of IMCRC, highlighted the importance of the studies in expediting the refinement of the design and manufacturing of the device.
“With Vaxxas planning to begin manufacturing the new needle-free vaccine delivery technology by early 2022, this IMCRC research project is vital to refine the device and fast-track its commercialisation process. These studies will assess the safety and acceptability of managing this technology within the healthcare community, with the results being fed back into the design and manufacturing process which is currently set up in Australia.”
Charles Ross, Head of Clinical Operations and Supply at Vaxxas said the collaboration with the University of Sydney brings world class practical vaccination and clinical trial experience to complement the capabilities of the Vaxxas research team.
“The distribution of vaccines and the requirements for successful vaccination uptake are critical success factors in a pandemic vaccination campaign. We are excited to be working with experts from the University of Sydney to assess the impact our revolutionary and simplified approach to
vaccination can have on the logistics and acceptability of the vaccination process in a close to realworld situation”.
Professor Robyn Ward, Pro Vice-Chancellor and Executive Dean of the Faculty of Medicine and Health, said the collaboration demonstrates the importance of supporting innovation in research.
“The COVID-19 pandemic has demonstrated, perhaps more than ever before, the crucial role our researchers play in innovation in the healthcare sector. Investment in projects such as this is vital and will ensure the continued strength of medical research in Australia,” said Professor Ward.
News & Trends - Biotechnology

Aussie biotech secures agreement with UQ and USyd to advance cardiovascular diseases research
Biotech News: Australian biotechnology company, Cartherics, developing immune cell therapies for the treatment of cancer, has entered into a Technology […]
MoreNews & Trends - MedTech & Diagnostics

NSW Health Secretary urges more focus on patient experience, drawing from her own heart valve disease journey
In a heart-warming event that united patients, their loved ones, representatives from patient organisations and employees, Edwards Lifesciences hosted its […]
MoreHuman Resources

‘To be an inclusive society, we need an embedded national strategy to combat systemic racism’, says Commissioner Sivaraman
Race Discrimination Commissioner Giridharan Sivaraman has welcomed the release of the Multicultural Framework Review by the Federal Government, calling it […]
MoreNews & Trends - Pharmaceuticals

Shingles vaccine lowers risk of dementia
Pharma News: A recombinant shingles vaccine which was added to Australia’s National Immunisation Program (NIP) in November last year, is […]
More